Resource for Reporting Potential Donor-Derived Disease Transmission Events (PDDTE)
Background
The OPTN Disease Transmission Advisory Committee (DTAC) considers issues related to the transmission of disease through organ transplantation. It examines the individual potential disease transmission cases reported to the OPTN in an effort to confirm transmissions where possible, and it reviews aggregate data on all reported cases to assess the risk of donor disease transmission in organ transplantation in the U.S. with the goal of providing:
- education and guidance to the transplant community toward preventing future disease transmission; and
- input in developing policy to improve the safety of organ donation through the reduction of donor-derived transmission events.
As part of this work, the DTAC may identify disease-transmission related patient safety issues to be addressed, as appropriate, by the OPTN.
This web page provides information regarding what and when to report to the OPTN's Improving Patient Safety reporting portal for members in the OPTN Computer System.
What is the Purpose of Reporting PDDTE?
PDDTEs submitted to the OPTN's Improving Patient Safety portal serve several purposes, including a common goal of enhancing patient safety.
- Reports allow for confirmation of recipient transplant program notification, and further recipient assessment and evaluation.
- The OPTN contractor’s patient safety team communicates with OPTN members to help facilitate communication between transplant programs, OPOs, and relevant governmental agencies.
- Reporting allows the OPTN contractor to collect information related to these events. Lessons learned from review of aggregate information collected from PDDTE may result in new policy or guidance that can be shared with transplant centers and OPOs to prevent future transmission events.
Circumstances Where Reporting a PDDTE is Required
In general, there are two levels of handling PDDTEs:
- Communication of the finding between the Host OPO and recipient Transplant Center Patient Safety Contact; or
- Communication of the finding between the Host OPO and recipient Transplant Center Patient Safety Contact and reporting of the case through the Improving Patient Safety Portal in the OPTN Computer System.
OPO Requirements:
When and if donor data become available to the host OPO after cross-clamp (i.e. a positive culture, new histopathology result confirming or suggestive of malignancy), OPTN policy requires the finding be communicated between the host OPO and recipient Transplant Center Patient Safety Contact(s) as soon as possible but no later than 24 hours following receipt of new data. As outlined in Policy 15.4.A: Host OPO Requirements for Reporting Post-Procurement Donor Results and Discovery of Potential Disease Transmissions, the host OPO must also report the following events to the Improving Patient Safety Portal:
- Any result indicating detection of Pathogen of Special Interest (POSI)
- Malignancy or other findings highly suggestive of malignancy
Recipient Transplant Center Requirements:
Recipient transplant centers are required to report potential unexpected discovery of disease or malignancy as outlined in Policy 15.5: Transplant Program Requirements for Communicating Discovery of Potential Transmission of Unexpected Pathogen, Disease or Malignancy. All findings that require reporting must be communicated to the host OPO, or to the host OPO and the OPTN Improving Patient Safety Portal, within 24 hours when there is substantial concern that the suspected or confirmed disease, malignancy, or infection could be from the transplanted organ.
Figure 1 below outlines what results must be reported to the host OPO or to the host OPO and the OPTN Improving Patient Safety Portal, when there is substantial concern for a donor-derived disease transmission.
Figure 1: Algorithm for recipient transplant center reporting of potential unexpected discovery of disease 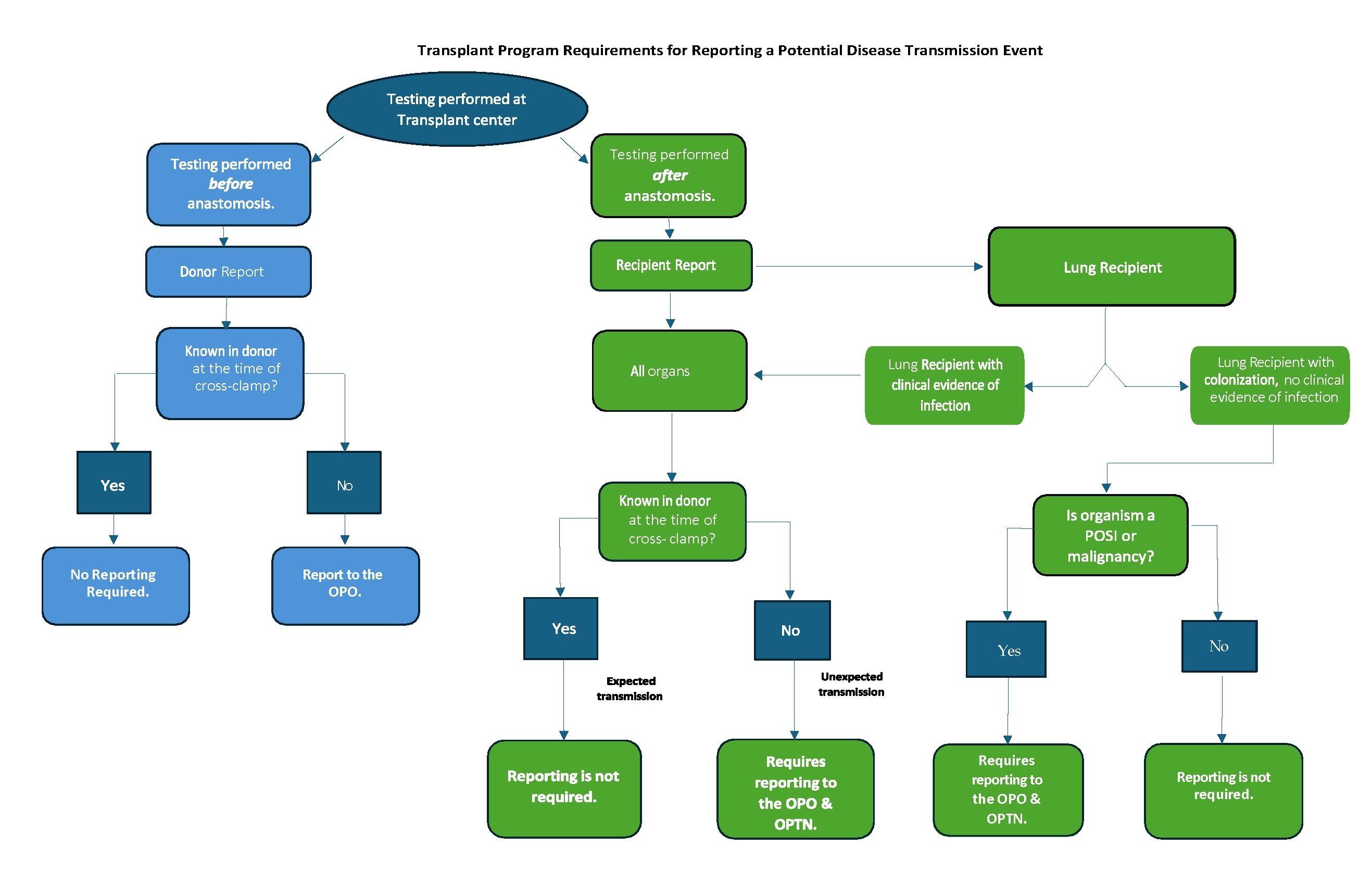
Full resolution image can be found here.
The following definitions should be used by recipient transplant centers when evaluating whether an event should be reported:
- Unexpected Transmission: A potential transmission is unexpected if the pathogen, disease, or malignancy was not known in the donor at the time of cross-clamp.
- Donor Report: Testing of the donor organ or donor specimen performed at the transplant center before anastomosis is considered a donor report.
- Recipient Report: Testing performed at the transplant center after anastomosis is considered a recipient report.
- Lung recipients with clinical evidence of infection versus Lung Recipients with evidence of colonization but not clinical evidence of infection:Lung transplant recipients undergo frequent airway sampling in the early post-transplant period, but not all organisms isolated from the respiratory tract represent true pathogens. Therefore, distinguishing between a lung recipient with clinical evidence of infection versus a lung recipient with evidence of colonization but not clinical evidence of infection is important to determine the need for reporting.
- Lung Recipient with clinical evidence of infection: Lung recipient who has an organism isolated from the respiratory tract or other site that directly contributes to the lung recipient’s illness based on the clinical judgement of the treating physician team.
- Lung Recipient with evidence of colonization but not clinical evidence of infection: Lung recipients not meeting the definition of “a lung recipient with clinical evidence of infection” are considered lung recipients with evidence of colonization but not clinical evidence of infection.
- Pathogen of Special Interest (POSI): Pathogens that must be reported to the OPTN Improving Patient Safety Portal, irrespective of donor or recipient illness
Additional recipient transplant center considerations:
- Malignancies suspected to be donor-transmitted are reported to the Improving Patient Safety Portal in the OPTN Computer System and separately reported using the Transplant Recipient Follow-up (TRF) form. All other malignancies, including post-transplant lymphoproliferative disorder (PTLD), are reported using the TRF form only.
- In rare circumstances, there may be concern for transmission of other conditions other than infectious disease or malignancy. The recipient care team should report a new, unexpected condition as a PDDTE if there is substantial concern that it may be donor-derived.
If you are unsure whether a specific situation should be reported as a PDDTE, it is recommended that you report it in order to promote patient safety.
It is recommended that OPO staff talk with their Medical Director if they need additional guidance regarding whether to report a PDDTE based upon final culture results or other new information learned post transplant.
Transplant programs may wish to pose questions related to whether a disease or malignancy could be donor-derived to infectious disease or oncology/pathology personnel and the recipient's attending transplant provider at their center. Transplant centers should always notify the OPO regarding the concern of potential donor-derived disease. This allows the Host OPO to contact any other recipient centers to determine if other recipients receiving organs from the same donor have developed similar symptoms or disease.
OPTN contractor Patient Safety Staff are available to assist you with questions during business hours or you may report directly into the Improving Patient Safety portal in the OPTN Computer System.
When to Report a PDDTE
If a PDDTE is suspected, the transplant program should notify the Host OPO and/or make a report to the Improving Patient Safety portal in the OPTN Computer System as soon as possible, and within 24 hours of suspecting donor-derived transmission—regardless of if all test results are finalized. This process alerts the OPO and allows them time to initiate contact with other transplant programs that have transplanted organs from the same donor to communicate information that may impact recipient testing, treatment or prophylaxis.
OPOs should communicate any new donor information to all recipient transplant programs as soon as possible and within 24 hours to allow recipient care teams to determine if additional testing, treatment or prophylaxis will benefit the recipient(s) as the result of this new information.