Continuous distribution - lung
Lung is the first organ type to work through establishing continuous distribution as its new framework for allocation. The work began with the Thoracic Committee in winter 2019. When the Lung Transplantation Committee formed in summer 2020, it continued the work on continuous distribution of lungs.
In fall of 2021, the Lung Transplantation Committee’s proposal Establish Continuous Distribution of Lungs opened for public comment.
The proposal received unanimous approval by the OPTN Board of Directors on Dec. 6, 2021. Plans for implementation are underway, and professional and patient education is in development.
The Lung Committee followed a series of steps to build the framework. Below you will find more information about what has been involved with each step of the process, and any results.
1. Identify attributes — status complete
The Lung Committee considered several attributes before deciding those that would contribute to the composite allocation score. They identified attributes and aligned them with NOTA and the OPTN Final Rule. Any attribute that is based in part on a candidate’s location or listing is only permitted to the extent required by another regulatory factor. The Committee began with the attributes that are in current policy, then considered additional attributes suggested during 2019 public comment. The committee has regularly updated the community about its progress.
- 2019 lung concept paper (PDF)
- Update on the continuous distribution of lungs project, request for feedback paper (PDF)
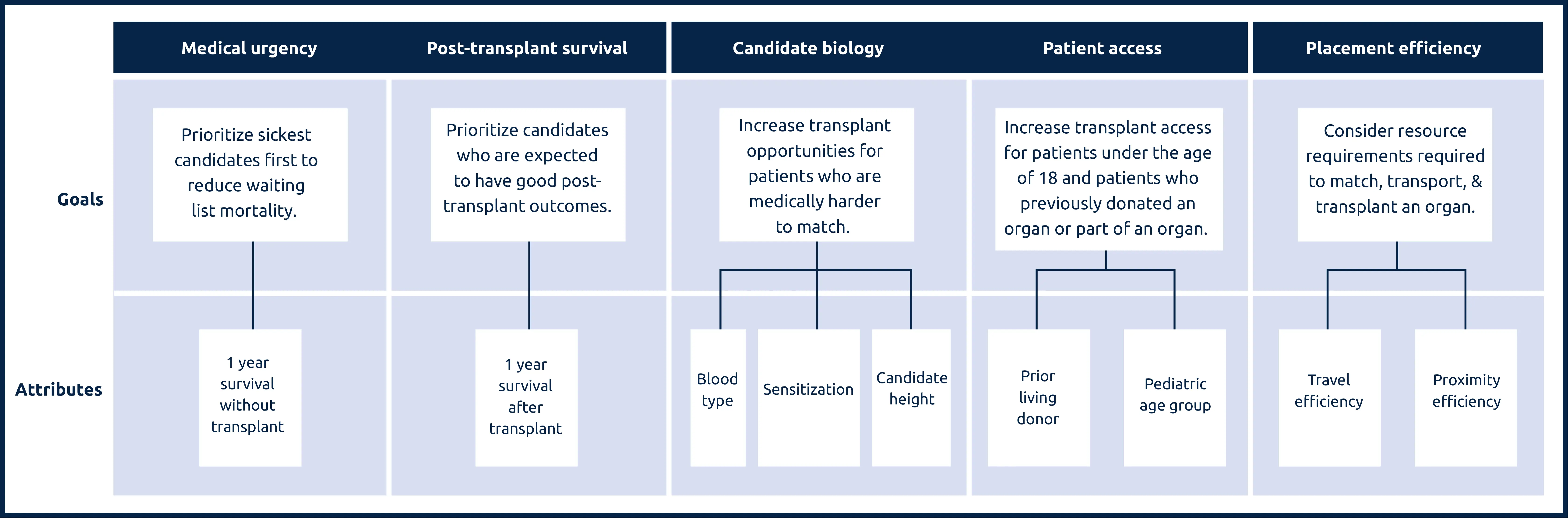
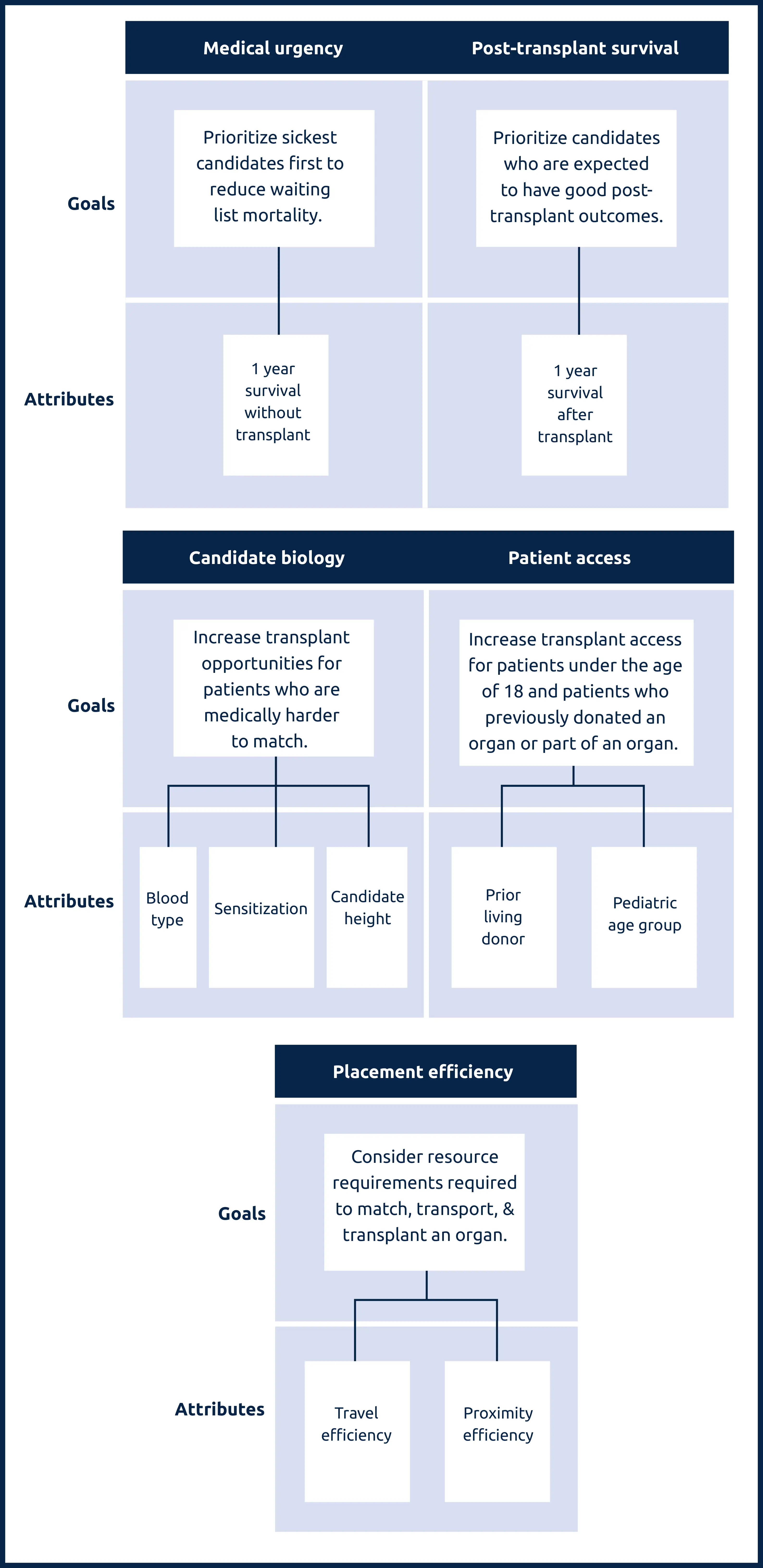
Lung attributes and the goals they support 

Medical urgency: Amount of risk to a candidate’s life or long-term health without receiving an organ transplant.
Post-transplant survival: A candidate’s likelihood of survival for five years after receiving a transplant.
Candidate biology: Medical characteristics of a candidate can make them harder to match. This can include a candidate’s blood type, their body’s sensitivity to accepting an organ, or their height.
Patient access: This addresses transplant access for candidates under the age of 18, as well as prior living donors, those who have previously donated an organ or part of an organ.
Placement efficiency: The amount of resources required to promote the efficient management of organ placement.
2. Build framework — status complete
This step involved converting lung’s existing allocation policy into the new framework through the following exercises.
Prioritizing attributes against each other
The specific weight of each attribute determined how much influence each attribute has toward the overall lung allocation score. This step had two parts:
a) Determining prioritization of attributes by collecting community input through a method called the Analytic Hierarchy Process, or AHP. Anyone can participate in the AHP exercises for continuous distribution, which will occur for all organ types. In an AHP exercise for continuous distribution, participants are shown pairs of attributes (blood type vs. distance, for example) that will be used to prioritize candidates. The AHP participant must decide, if all else is considered equal, which of the two attributes is more important than the other when prioritizing a candidate for an organ.
b) Comparing attributes to a “current state” through a revealed preference analysis. A revealed preference analysis involves looking at how multiple decisions have been made to reveal mathematical trends. For example, how important was distance compared to waiting time when a decision was made between two candidates? The revealed preference analysis for lung took the existing system and created a baseline to be measured against.
Converting attributes into points
For each attribute that was considered toward the overall score, the Lung Transplantation Committee decided how to assign points to candidates according to differences in the attribute.
Conducting sensitivity analysis
A sensitivity analysis is used by statisticians to change a single variable slightly to measure the impact on an outcome. For continuous distribution of lungs, a sensitivity tool was built to evaluate these variables. For example, if a change was made to the weight of any attribute, the new match run would be shown as the outcome.
Developing the composite score
Developing the composite score involved a combination of decided weights and rating scales. Among other resources, the committee utilized analyses from mathematical optimizations to assists in this step.
3. Modeling and analysis — status complete
At this phase, the Scientific Registry of Transplant Recipients (SRTR) took proposed allocation policies and modeled them to determine the impact on candidates. These results were produced in a report to help identify any potential unintended consequences or harmful outcomes for these example groups. These results estimated the benefit of the new proposal and informed any needed improvements. The modeling request for the SRTR was submitted in winter 2020.
- SRTR modeling results:
- Round 1 — (PDF) | Round 2 — (PDF) | Round 2 Addendum — (PDF)
- The impact of extending follow-up for the PTAUC model from 1 year to 5 years after transplant (PDF)
NOTE: It was determined in July 2023 that these modeling results did not incorporate screening for incompatible blood types in the simulation. For more information, refer to the proposal Modify Lung Allocation by Blood Type.
4. Public comment on policy proposal — status complete
This step involved considering community input, modeling and analysis, and committee project work, as well as proposing a new composite score as a policy proposal for public comment.
Read the 2021 proposal (PDF) to learn more about the changes to the lung allocation scoring process and the lung exception review process. The public comments on this proposal are here.
5. Board approval — status complete
The Board of Directors reviewed the proposal, OPTN and SRTR materials, and public comments, and considered the proposal in light of the requirements of the OPTN final rule. The board unanimously approved the proposal to establish continuous distribution of lungs on Dec. 6, 2021.
- Read the board briefing paper (PDF)